Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Interleukin33-NFκB Axis Mediated DNA Damage Repair in Neurons: Does Its deficiency Initiate a Path toward Late-Onset Alzheimer’s Disease?
*Corresponding author:Yahuan Lou, Department of Diagnostic Sciences School of Dentistry, University of Texas Health Science Center at Houston, Houston, TX 77054, USA.
Received: February 18, 2022; Published: March 23, 2022
DOI: 10.34297/AJBSR.2022.15.002170
Abstract
Sporadic late-onset Alzheimer’s disease remains a medical mystery. Recent studies, including ours, suggest that abnormal aging process in neurons may be responsible. Several pathological mechanisms, which may lead to chronic development of late-onset Alzheimer’s disease, have been explored. One of them is accumulation of DNA double-strand damage (DSB) in neurons after middle age due to abnormal aging or failed DSB repair. Because of non-proliferative nature, aged neurons must be rejuvenated to keep their functionality for nearly whole lifespan. DNA integrity would be critical to ensure rejuvenation. Our recent study further showed that interlukin33-ST2-NFκB axis in neurons is required to initiate DSB repair in neurons. Thus, deficiency of this axis in neurons not only impairs DSB repair but also disrupts neuronal rejuvenation, leading to chronic development of Alzheimer’s like disease in aged mice. It would be worthwhile to investigate if it is the case in humans.
Introduction
Late-Onset Alzheimer’s Disease and Failed Rejuvenation of Aged Neurons
Late-onset Alzheimer’s disease (LOAD) is an increasing socioeconomic burden worldwide. Unfortunately, its cause remains a medical mystery. Neurons are largely non-proliferative, and they usually cannot be replaced through cell division. Therefore, rejuvenation of aging neurons is an essential way for keeping brains’ functionality up to old age. Recently, mounting evidence supports that LOAD may be a result from abnormal neuronal aging due to either accelerated aging process or impairment in rejuvenation of aged neurons [1-5]. Rejuvenation mechanisms in neurons include repair of aging-associated DNA damage, autophagic digestion of metabolic molecules and/or damaged neuronal proteins such as abnormal tau, and glymphatic drainage of abnormal neuronassociated proteins/wastes such as amyloid [6-10]. In fact, linkages between defective rejuvenation mechanisms and LOAD have been either partially demonstrated in several animal models, or statistically established by clinical observations. In familial earlyonset type, Alzheimer’s characteristics (i.e., tauopathy and amyloid plaques) are caused by structural alteration of those proteins due to mutations in genes such as APP and MAPT. However, it remains a puzzle why failed rejuvenation mechanisms would lead to LOAD with nearly identical characteristics to familial type of Alzheimer’s disease. Although a single rejuvenation mechanism can be well linked to LOAD, so far animal models with deficiency in one single mechanism (e.g., autophagy or glymphatic) do not develop any Alzheimer’s like symptoms in mice without mutant human APP or MAPT transgenes. Those recent studies suggest requirement of deficiency in multiple rejuvenation mechanisms for LOAD developing. It is possible that there is a potential upstream regulatory factor or system that governs overall neuronal rejuvenations, and thus, its deficiency may impair all neuronal rejuvenations.
Unrepaired DNA Double-Strand Breaks (DSB) In Aged Neurons May Impair Their Rejuvenation
Similar to other cells, accumulation of oxidative damages in various biomolecules is an important part of neuronal aging. We have discovered a sudden oxidative damages surge in the cortical/ hippocampal neurons at middle age in mice (40 weeks ≈ human 46 years); such damage surge does not occur in any glial cells, or other organs [11,12]. Interestingly, sudden aging in brains at middle age has also been described in humans [13,14]. Oxidative damages in DNA lead to double-strand breaks (DSB), which must be repaired immediately. Otherwise, accumulated DSBs trigger genomic instability, which incapacitates stressed neurons to rejuvenate themselves or die through apoptosis. Accumulation of DSBs in Alzheimer’s brains have been reported [1,2,15-19]. It may initiate a chronic path from neuronal malfunction at middle age to neurodegeneration and dementia at old age. If it is the case, middle age may be a time window for early diagnosis or intervention of LOAD before irreversible neuron loss. DSBs-related genomic instability in neurons has been postulated as a potential cause for LOAD. There are two possible mechanisms that cause accumulation of DSBs in neurons: accelerated generation of DSB, or failure in DSB repair. DSB repair mechanisms have been well elucidated. Our study demonstrated that failure in DSB repair may be a major reason for DSB accumulation in neurons after middle age, at least in mice [11]. Which molecules or pathways regulate or initiate DSB repair in oxidatively stressed neurons?
Interleukin33-ST2-NFkB axis is critical for initiating DSB repair in neurons
Interleukin33 (IL33) is a member of the interleukin1 cytokine family and was first discovered as a nuclear protein [20]. After cleavage of nuclear IL33, a mature cytokine domain (cIL33) is released, which, in turn, binds to receptor ST2 to trigger NFκB pathway [21]. It was previously considered a multifunctional cytokine in immune defense/response. However, constitutive expression of IL33 in a wide range of tissues or cells including the brain suggests its potential roles beyond immune system [22-25]. It may be involved in the injury healing in central nervous system and other diseases such as cardiovascular diseases [26-29]. IL33 has been genetically linked to human Alzheimer’s disease and shows a beneficial effect in a mouse Alzheimer’s model [30,31]. Our previous study has demonstrated role of IL33 in tissue homeostasis in degenerative ovarian tissue [32]. Importantly, we also discovered a high level of IL33 expression in astrocytes; up to surprisingly 70% of astrocytes in certain brain regions would contain nuclear IL33 at old age, with constant release of cIL33 in brains [11]. In addition, we not only revealed neuronal expression of ST2, but also detected activation of NFκB pathway in neurons in NFκB/luciferase reporter transgenic mice. Thus, there exists an IL33-ST2-NFκB axis in neurons in brains [Fig. 1]. Importantly, IL33-NFκB axis has been detected in brains of human Alzheimer’s disease as well [33]. Using IL33 deficient (Il33-/-) mice, we discovered that astrocyte IL33 (but not exogenous IL33) is essential for neurons to control sudden oxidative damages at middle age, and its deficiency in mice caused abnormal tau deposition and late-onset neurodegeneration in the cerebral cortex and hippocampus, accompanied by Alzheimer’slike cognition and memory impairment [11,12]. Those observations raised an interesting question: Is astrocyte IL33 governs overall neuronal rejuvenation by regulation of DSB repair after oxidative damage surge? Our two sets of discoveries answer this question. First, IL33-deficient mice show a rapid accumulation of DSBs in neurons after the oxidative damage surge (Figure 2A). Second, DSB accumulation is due to failure in initiating DSB repair [Figure 2B]. Thus, neuronal IL33-ST2-NFκB axis is critical for initiation of DSB repair. Involvement of NFκB pathway in DSB repair in various tissues including aged brains has been reported [34-36]. At this moment, we are still exploring how NFκB pathway is involved in regulation of DSB repair in stressed neurons. Neuroinflammation has recently been considered as a cause or a promotor for LOAD. However, it is an unlikely scenario that IL33 involved in LOAD through promoting neuroinflammation.
Defective interleukin33-ST2-NFkB axis: a novel model for LOAD
Our study has shown that IL33 deficiency first fails initiation of DSB repair. Accumulation of DSBs in neurons, in turn, disrupts multiple rejuvenation mechanisms such as neuronal autophagy and glymphatic drainage [11,37]. As we have discussed earlier, defects in those mechanisms have been linked to LOAD. It is not surprised that IL33 deficient mice develop chronic neurodegenerative diseases, following a similar course to, and shares hallmarks with sporadic LOAD: massive synapse and neurite loss in the cortex and hippocampus after middle age, abnormal tau deposition in neurons and chronic neurodegeneration, and finally cognition impairment and memory loss at late life after a long asymptomatic period [11]. Although two types of Alzheimer’s disease display similar pathological characteristics (i.e., tau deposition and amyloid plaques), those in LOAD are unrelated to genomic mutation. From this point of view, neurodegenerative disease in IL33-deificency mice mimics LOAD better than other genomic mutation-based Alzheimer’s models. In fact, there are only a few, if any, animal models that “naturally” develop tau deposition without MAPT mutations. One may argue that IL33 deficient mice do not develop Amyloid plaques, one of the most important hallmarks of Alzheimer’s disease. In fact, unlike humans or other species, murine APP gene lacks structural base for formation of plaques [38]. However, abnormal tau deposition in IL33-deficienct brains may have indicated that lack of neuronal rejuvenation has created an internal environment that promotes protein denaturation, accumulation, and aggregation. We have shown that reduced glymphatic drainage in IL33 deficient mice accelerates abnormal tau accumulation in brains [37]. We are currently testing if it is the case for amyloid plaque in IL33 deficient human APP transgenic mice.
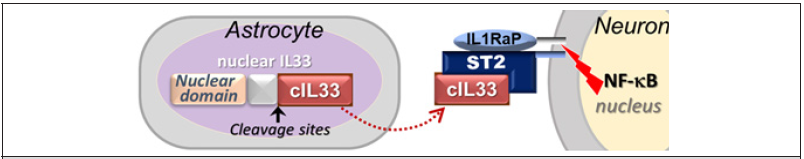
Figure 1: Interleukin33-ST2-NFκB Axis in neurons. IL1RaP is common co-receptor for IL1 family cytokine.
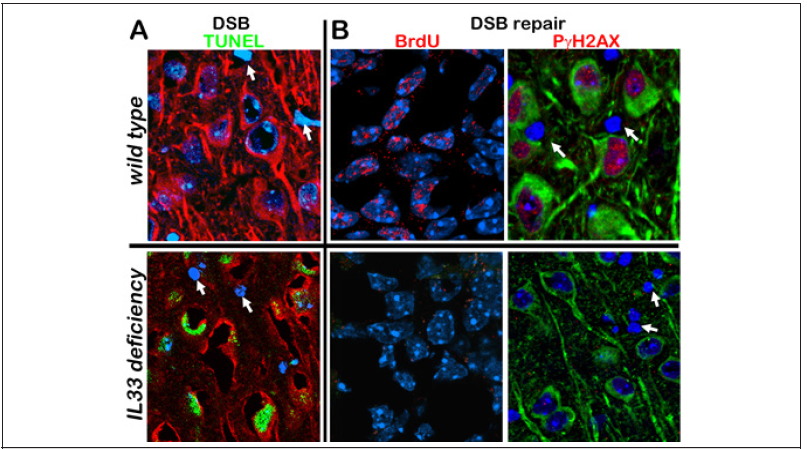
Figure 2: DNA double strand breaks (DBS) and their repair in neuron after oxidative damage. (A) Accumulation of DSBs (green) in neurons in IL33 deficient mice. In contrast, wild type neurons do not have any DSBs. (B) DSBs repairing occurs in neurons in wild type mice, but not in IL33 deficient mice. DSB repair is detected by BrdU incorporation (red) and by expression of repair initiating protein PγH2AX (red). Note that both DSB accumulation and DSB repair are absent in glial cells (arrows).
Our data, together with many others, allow us to give the following scenario for development of LOAD due to defective IL33- NFκB axis in neurons [Figure 3]. In normal individuals, immediate repair of DSBs during oxidative surge ensures normal rejuvenation process for stressed neurons toward a full recovery. On the other hand, accumulation of unrepaired DSBs due to IL33-NFkB deficiency disrupts all subsequent rejuvenation mechanisms. Massive DSBs should otherwise have induced apoptosis in any other types of cells, but not in those neurons [11] [Figure 2]. It raises an interesting question why neurons with massive DSBs due to failed DSB repair mechanism do not go to apoptosis, or, how they can survive for a long time. We have not had a full answer for it. Our recent study in IL33 deficiency model revealed a strong re-expression of apoptozole protein Apaf1, which has been silenced in mature neurons, after oxidative damage surge, together with expression of several pro- and anti-apoptotic genes. It suggests a death and life struggle by stressed neurons. Complicated interactions among those proteins may avoid apoptosis in neurons but with a significant price, i.e., loss of their functionality by shrinking or withdrawing neurites and synapse as seen in early human LOAD and middle aged IL33 deficient mice [11]. Since those neurons never recover, they eventually die or lose all functions.
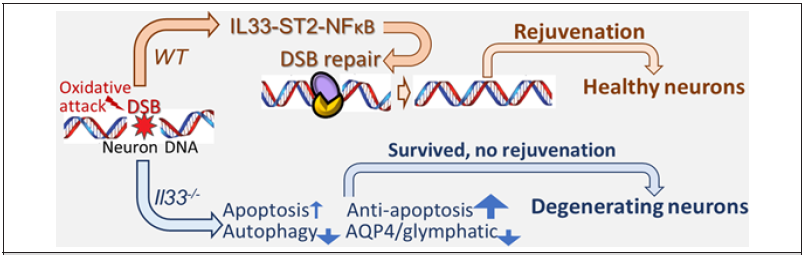
Figure 3:IL33-ST2-NFκB axis regulates DSB repair. DSBs are repaired stressed neurons to keep genome integrity; stressed neurons are fully rejuvenated. On the other hand, unrepaired DSBs in IL33 deficient (IL33-/- ) neurons cause genomic instability and disrupts neuronal rejuvenation. Although anti-apoptotic mechanism avoids their death, neurons chronically degenerate with loss of functions.
Acknowledgements
This work was supported by NIH R21AG067311 (to YL), NIH R01DK077857 (to YL) and NIH R01HD049613 (to YL).
Conflict interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anderson AJ, Su H, Cotman CW (1996) DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci 16(5): 1710-1719.
- Coppede F, Migliore L (2009) DNA damage and repair in Alzheimer's disease. Curr. Alzheimer Res 6(1): 36-47.
- Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19(8): 983-997.
- Frake RA, Ricketts T, Menzies FM, Rubinsztein DC (2015) Autophagy and neurodegeneration. J Clin Invest 125(1): 65-74.
- Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, et al. (2015) DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun 6: 8897.
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, et al. (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA 101(7): 2070-2075.
- Barnett A, Brewer GJ (2011) Autophagy in aging and Alzheimer's disease: pathologic or protective? J Alzheimers Dis. 25(3): 385-394.
- Swerdlow RH (2011) A Brain aging, Alzheimer's disease, and mitochondria. Biochim Biophys Acta 1812(12): 1630-1639.
- Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16(6): 345-357.
- Wyss Coray T (2016) Ageing, neurodegeneration, and brain rejuvenation. Nature 539(7628):180-186.
- Carlock C, Wu J, Shim J, Moreno-Gonzalez I, Pitcher M, et al. (2017) Interleukin33 deficiency causes tau abnormality and neurodegeneration with Alzheimer-like symptoms in aged mice. Trans Psychiatry 7(8): e1164.
- Lou Y (2021) Role of interleukin33 in rejuvenation of aged neurons and age-related dementias. Neurosci Insights 16: 26331055211030251.
- Bender AR, Völkle MC, Raz N (2016) Differential aging of cerebral white matter in middle-aged and older adults: A seven-year follow-up. NeuroImage 125: 74-83.
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, et al. (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22(4): 581-594.
- Reid DA, Reed PJ, Schlachetzki JCM, Nitulescu II, Chou G, et al. (2021) Incorporation of a nucleoside analog maps genome repair sites in postmitotic human neurons. Science 372(6537): 91-94.
- Mark A. Lovell, William R. Markesbery (2007) Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res 35(22):7497-7504.
- Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, et al. (2020) Contributions of DNA Damage to Alzheimer's Disease. Int J Mol Sci 21(5): 1666.
- Shanbhag NM, Evans MD, Mao W, Nana AL, Seeley WW, et al. (2019) Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol Commun 7: 77.
- Pao PC, Patnaik D, Watson LA, Gao F, Pan L, et al. (2020) HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat Commun 11: 2484.
- Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, et al. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163(1): 69-79.
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23(5): 479-490.
- Yasuoka S, Kawanokuchi J, Parajuli B, Suzumura A (2011) Production and functions of IL-33 in the central nervous system. Brain Res 1385: 8-17.
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, et al. (2012) Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 188(7): 3488-3495.
- Wicher G, Husic E, Nilsson G, Forsberg-Nilsson K (2013) Developmental expression of IL-33 in the mouse brain. Neurosci Lett 555: 171-176.
- Martin NT, Martin MU (2016) Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 17(2): 122-131.
- Li M, Li Y, Liu X, Gao X, Wang Y (2012) IL-33 blockade suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neuroimmunol 247(1-2): 25-31.
- Stojanovic B, Milovanovic J, Arsenijevic A, Milovanovic M, Arsenijevic N (2014) IL-33/ST2 axis mediates resistance to EAE by promoting regulatory B and tolerogenic dendritic cells. Neuroimmunol 275(1-2): 11-12.
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J (2015) The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS Injury. Neuron 85(4): 703-709.
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, et al. (2007) IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 117(6): 1538-1549.
- Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, et al. (2009) Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer's disease. Mol Psychiatry 14(11): 1004-1016.
- Fu AK, Hung KW, Yuen MY, Zhou XD, Mak S, et al. (2016) IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc Natl Acad Sci USA 113(19) : E2705-2713.
- Wu J, Carlock C, Zhou C, Nakae S, Hicks J, et al. (2015) Interleukin33 is required for disposal of unnecessary cells during ovarian atresia through regulation of autophagy and macrophage migration. J Immunol 194(5): 2140-2147.
- Xiong Z, Thangavel R, Kempuraj D, Yang E, Zaheer S, et al. (2014) Alzheimer's disease: evidence for the expression of interleukin-33 and its receptor ST2 in the brain. J Alzheimers Dis 40(2): 297-308.
- Volcic M, Karl S, Baumann B, Salles D, Daniel Peter, et al. (2012) NF-κB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res 40(1): 181-195.
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, et al. (2012) NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 122(7): 2601-2612.
- Kraft D, Rall M, Volcic M, Metzler E, Groo A, et al. (2015) NF-κB-dependent DNA damage-signaling differentially regulates DNA double-strand break repair mechanisms in immature and mature human hematopoietic cells. Leukemia 29(7): 1543-1554.
- Wu J, Shim J, Carlock C, Moreno-Gonzalez I, Barichello T, et al. (2021) Interleukin33 regulates aquaporin4 expression in astrocytes and glymphatic drainage of abnormal tau from brains. Mol Psychiatry 26(10): 5912-5924.
- Gotz J, Ittner LM (2008) Animal models of Alzheimer’s disease and frontotemporal dementia. Rev Neurosci. 9(7): 532-544.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.